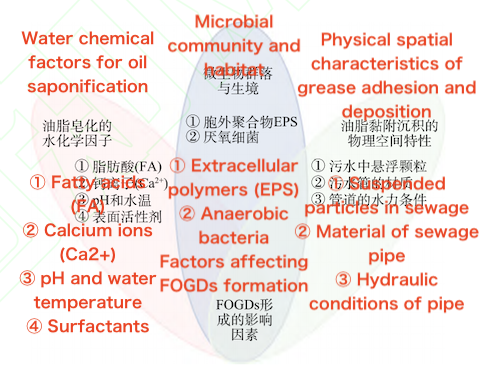
Hydrochemical factors were the first to attract attention in research on FOGD formation and, as a result, have been relatively well studied. The primary hydrochemical factors currently under investigation include fatty acids (FA), calcium (Ca²⁺) and other metal ions, surfactants, pH, and water temperature.
(FA) are the main reactants in the saponification process that leads to the formation of FOGDs. The FA content in FOGDs found in sewer pipes typically ranges from 78.5% to 91.4%. Studies have shown a positive correlation between FA content and the amount of FOGDs formed. Lasmin et al., using Fourier Transform Infrared Spectroscopy-Attenuated Total Reflectance (FTIR-ATR), identified three characteristic absorption bands in FOGDs: the signature peak of calcium-based fatty acid salts, a strong metal–oxygen bond vibration peak (around 670 cm⁻¹), and a hydroxyl stretching vibration peak (around 3400 cm⁻¹). These spectral features confirm that FA is a key component of FOGDs. He et al. analyzed 19 FOGD samples collected from 23 urban sewer pipes across the United States. The results showed that 16 samples (84%) had FA contents exceeding 50%. In addition, calcium was detected in the samples at an average concentration of 4,255 mg·L⁻¹, suggesting that FOGDs primarily exist in the form of fatty acid metal salts.
The degree of saturation and type of fatty acids (FAs) also influence the formation of FOGDs. Lasmin et al. investigated the solid yield from saponification reactions using the same calcium source, saturated beef tallow, and unsaturated rapeseed oil under identical pH and temperature conditions. The results showed that the saponification rate of rapeseed oil reached 42.9%, while that of beef tallow was only 19.1%, indicating that unsaturated fatty acids (UFAs) may be more favorable for FOGD formation. Del et al. studied the saponification reactions of catering wastewater derived from different fat and oil sources, as well as the characteristics of the resulting solids. The results demonstrated that the yield of calcium soap varied significantly depending on the type of fat or oil. The order of calcium soap yield from lowest to highest was: linoleic acid, lauric acid, oleic acid, and palmitic acid. Similarly, the order based on FA type was: long-chain polyunsaturated fatty acids, medium-chain saturated fatty acids, and long-chain monounsaturated fatty acids. A higher ratio of oleic acid to palmitic acid led to increased FOGD formation during saponification. Compared with saturated fatty acids (SFAs), calcium salts formed from unsaturated fatty acids and calcium ions exhibit stronger adhesion due to their distinctive double-bond structures. This enhanced adhesion allows them to more easily attach to the surfaces of concrete pipes, thereby promoting the generation and accumulation of FOGDs.
Ca²⁺ is the primary metal ion involved in the saponification reaction that leads to the formation of FOGDs. Variations in its source, type, and concentration can significantly influence the physical properties of the resulting deposits. Studies have shown that saponified sediments formed with calcium sulfate (CaSO₄) and calcium hydroxide (Ca(OH)₂) tend to have coarse, granular characteristics, while those formed with calcium chloride (CaCl₂) are smoother and more viscous. In sewage, Ca²⁺ mainly originates from the influent and from the corrosion of concrete pipes. This calcium not only raises the melting point of FOGDs (up to 24°C) and reduces their water content (promoting solidification), but also significantly increases the overall formation of FOGDs. Gross et al. analyzed actual sewage pipe samples and found that, in addition to Ca²⁺, FOGDs also contain other metal ions such as K⁺, Na⁺, and Fe²⁺. However, because Ca²⁺ exhibits preferential binding in the saponification reaction (compared to Fe²⁺), the majority of FOGDs in pipes consist of fatty acid metal salts formed primarily with Ca²⁺.
It is also worth noting that adding an appropriate amount of Ca²⁺ can help mitigate its inhibitory effect on anaerobic digestion by reducing the concentration of long-chain fatty acids (LCFAs) in FOGDs, thereby enhancing CH₄ production. Experimental data show that when 0.1% Ca²⁺ is added to the reactor, biomethane production is promoted; increasing the Ca²⁺ concentration to 0.3% results in a fivefold increase in CH₄ production, and at 0.5%, CH₄ production increases by up to six times. However, excessive Ca²⁺ concentrations (>0.5%) may lead to the precipitation of phosphate and carbonate, which not only inhibits methanogenic activity but also alters the physical and chemical properties of FOGDs, negatively affecting their degradation behavior.
In sewer systems, pH is one of the key factors influencing the formation of FOGDs (fat, oil, and grease deposits). On one hand, pH affects the solubility of Ca²⁺ in water, thereby altering the availability of free Ca²⁺ and OH⁻ ions involved in fat hydrolysis and saponification reactions. Under neutral to alkaline conditions, a higher pH increases the availability of free Ca²⁺, accelerates the saponification reaction, and leads to the formation of more saponified solids and, consequently, more FOGDs. On the other hand, changes in pH also impact the degree of concrete pipe corrosion, which in turn affects the release and concentration of Ca²⁺ in the wastewater—further influencing FOGD formation.
Studies have shown that under acidic conditions (pH = 3), the release of Ca²⁺ from concrete can reach 381 mg/L, which is 3.0 times and 5.3 times higher than that at pH 6 and pH 8, respectively. Acidic environments not only accelerate concrete corrosion but also cause more Ca²⁺ to leach into the water, further promoting FOGD formation. Therefore, scientifically regulating the pH of wastewater can be an effective way to control the generation and accumulation of FOGDs. Similarly, wastewater temperature also affects the FOGD formation process by influencing the solubility of calcium sources. At elevated temperatures (≥45°C), solid fats dissolve more readily, the hydrolysis rate of fatty acids (FAs) increases, and the saponification reaction is accelerated, leading to faster and greater FOGD formation. Moreover, high temperatures can cause evaporation and concentration of the wastewater, increasing the concentrations of FA and Ca²⁺, and further exacerbating FOGD accumulation.
Oily wastewater discharged from households and the catering industry often contains a certain concentration of alkaline surfactants. The widespread use of these highly alkaline surfactants (pH > 10) can promote saponification reactions by acting as oxidizing agents and increasing the pH, thereby contributing to the formation of FOGDs. The distinct dual nature of surfactants, which contain hydrophilic groups such as hydroxyl, carboxyl, and carbonyl that dissolve easily in water, and lipophilic hydrocarbon chains that dissolve in oils, reduces the surface tension of the aqueous solution and makes it easier for oils and microorganisms to adhere to the surface of FOGDs.
Furthermore, surfactants facilitate the hydrolysis and acidification of oils in wastewater, enhance the microbial bioavailability of oils, and alter the substrate structure of anaerobic fermentation within sewer systems, thereby promoting the generation of FOGDs. In addition, surfactants can break down large fat globules into smaller fragments, increasing the surface area available for microbial degradation via enzymatic activity. This not only accelerates the reaction rate but also contributes to some extent to the formation and accumulation of FOGDs.
Suspended particles in sewage pipes can act as carriers for lipid attachment, thereby influencing the formation of FOGDs (fat, oil, and grease deposits). Multiple surveys and analyses have shown that the concentration of FOGDs in sewer systems increases significantly in areas with high levels of suspended solids and dense concentrations of food service establishments. Williams et al. noted that the continuous capture of suspended particles by lipid polymers composed of fatty acids (FAs), triglycerides, and fat-soluble hydrocarbons is a key mechanism driving the gradual accumulation of FOGDs. Further research by Otsuka et al. confirmed that FOGDs are likely to form pipe blockages only when both FAs and suspended particles are present. In the absence of suspended particles, even with the presence of FAs, FOGD formation is minimal and pipe blockages do not occur.
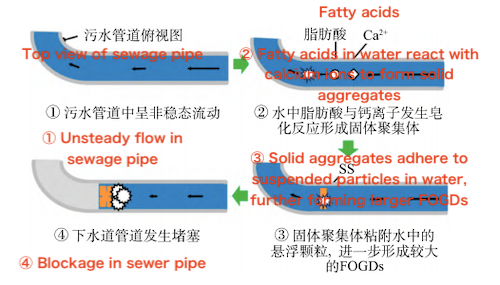
Currently, commonly used sewage pipe materials include reinforced concrete, metal, and plastic. Generally, FOGDs are more likely to adhere to pipe surfaces with higher roughness. Compared to smooth materials such as plastic and metal, reinforced concrete pipes not only have a rougher surface but are also more prone to corrosion in complex wastewater environments. This corrosion releases calcium ions (Ca²⁺), which further promote saponification reactions and accelerate the formation and accumulation of FOGDs. Additionally, larger pipe diameters and lower flow velocities favor the deposition of FOGDs. In contrast, in pipes made of non-concrete materials like plastic, the primary source of Ca²⁺ is the water itself. As a result, the formation and accumulation of FOGDs in these pipes is significantly lower than in reinforced concrete pipes.
The hydrodynamic conditions within sewage pipes further influence the formation and deposition of FOGDs by affecting the migration and interaction of lipids and particulate matter. When the water flow rate is below 0.6 m/s, the deposition effect prevails, and particulate matter is more likely to settle at the bottom of the pipe. However, when the flow rate exceeds 0.6 m/s, the scouring effect increases, and sediments are more easily carried away by the flow. In specific areas of the pipeline, such as manholes, sagging bends, or inflection points, the flow rate is lower, leading to a higher accumulation of solid matter. The slow, viscous laminar flow in such regions promotes the attachment of particles, such as grease and oil, to the pipe walls, increasing the residence time of the material and facilitating deposition. Additionally, the low turbulence level in the pump pool area of the sewage system also provides favorable conditions for the formation of FOGDs.
Del et al. also discovered that the calcium soap present in FOGDs exhibits distinct rheological properties. At 25°C, when the shear rate increases from 1.0 s⁻¹ to 800 s⁻¹, the apparent viscosity decreases from 1.56 Pa·s to 0.11 Pa·s. This demonstrates that FOGDs behave differently from traditional sediments, with their critical shear stress and starting flow rate differing from those of general particles. Therefore, scientifically regulating the water flow velocity and shear force within the pipeline can help effectively manage and reduce the formation and accumulation of FOGDs.
Compared to physical and chemical factors such as sewage water composition and the physical spatial characteristics of oil and fat adhesion, biological factors, including microbial communities and habitats, have a more complex impact on the formation of FOGDs. On one hand, microorganisms in sewage can form biofilms on the surface of particles and pipe walls by secreting extracellular polymeric substances (EPS). These biofilms not only increase the viscosity between particles and walls but also alter the surface chemical properties, enhancing the anti-scouring ability of the sediments. EPS can be classified into two categories based on their characteristics: bound and dissolved. Bound EPS consolidates inorganic particles and oils together through physical bridging, while dissolved EPS can influence the growth and renewal of biofilms by changing the substrate content in water.
While moderate shear force and flow rates can increase the diffusion rate of substances in the biofilm, promoting the shedding of aged biofilms and the renewal of biofilms, excessively high flow rates may inhibit biofilm activity and affect its adhesion to FOGDs. On the other hand, specific microbial communities in the pipeline possess certain biodegradation capabilities for FOGDs. For instance, microorganisms from the genus Syntrophomonas and the family Syntrophomonadaceae can effectively degrade medium- and long-chain fatty acids. Under anaerobic conditions in sewage pipes, these microorganisms may adhere to pipe walls and sediments, forming a biodegradation process dominated by anaerobic biochemical reactions. This process can not only promote the reproduction and proliferation of functional microorganisms but also adsorb oils and inorganic particles, leading to the formation of more FOGDs. Additionally, the enzymes released by anaerobic bacteria on the biofilm can hydrolyze and hydrogenate oleic acid into palmitic acid, accelerating the coagulation of FOGDs.
The formation of FOGDs is a complex process that involves not only chemical reactions but also multiple stages such as physical transport and biological accumulation. These factors work together to determine the generation pathway and characteristics of FOGDs.
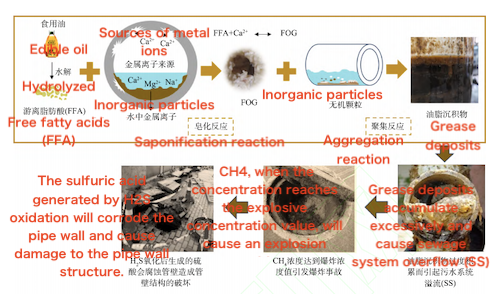
The chemical formation mechanism of FOGDs primarily involves the interaction between metal ions (Ca²⁺), free fatty acids (FFA), and fats (or oils) in catering and oily wastewater. The saponification reaction between FFA and Ca²⁺ is the core reaction, forming the initial core of FOGDs. This process has been confirmed through Fourier transform infrared spectroscopy (FTIR) analysis. Subsequently, the excess Ca²⁺ in the sewage compresses the double electric layer of the FFA molecules, causing the system to destabilize. Based on the formed FOGDs core, under the combined action of van der Waals forces and electrostatic repulsion, the system continuously adsorbs unreacted FFA, Ca²⁺, and organic debris in the wastewater, gradually forming a larger-scale FOGDs structure.
The physical formation mechanism of FOGDs is mainly reflected in their migration, deposition, and flushing behavior within sewage pipes. When FFAs in oily wastewater react with metal ions such as Ca²⁺ through saponification, solid aggregates of a certain size are formed. These aggregates often attach to suspended particles as the sewage flows downstream, forming larger grease deposits. Under high-flow conditions, these sediments are carried to sewage pumping stations or wastewater treatment plants; in low-flow areas, however, the aggregates are more likely to settle and eventually form a stable FOGDs sediment layer through adhesion and consolidation. It is worth noting that, compared with traditional sediments, FOGDs exhibit high water content, high volatility, low density, high yield strength, and a wide melting point distribution. Their hydrodynamic response and formation process within pipelines differ significantly from those of conventional sediments. For instance, the critical shear stress required to initiate the scouring of FOGDs is much higher than that of ordinary sediments, making them more difficult to remove and reducing the efficiency of hydraulic flushing.
Functional microorganisms commonly found in sewage pipes also play a role in the formation of FOGDs. Microorganisms that attach to oil particles and suspended solids can proliferate and form biofilms, thereby encapsulating and stabilizing the developing FOGDs. The adhesive effect of biofilms and extracellular polymeric substances (EPS) further enhances the structural integrity of FOGDs and increases their resistance to scouring. However, under strong hydrodynamic forces, these mature and solidified FOGDs, together with the attached biofilms, may still detach from the pipe walls or bottom, re-enter the sewage transport system, and contribute to new sedimentation processes. Additionally, the anaerobic microbial environment commonly present in sewage pipelines can selectively enrich certain functional bacteria, leading to the release of gases such as CH₄ and H₂S. These biological processes further influence the formation pathways and physical properties of FOGDs.
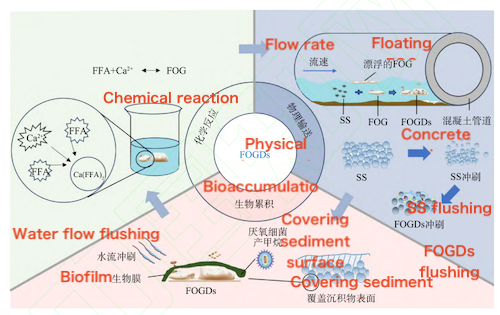
Figure 5 The formation mechanism of FOGDs